Web 🎯 want to ace chemistry? Percentage isotopic abundance practice means progress boost your grades with free daily practice questions.
3 Isotope Percent Problem Science, Chemistry, Isotopes ShowMe
Calculating Percent Abundance Of Isotopes Worksheet. Web in is worksheet, we will praxis calculating percentage isotopic abundances from the relative atomic ground and isotopic masses. This average atomic mass away elements is charged by: Percentage isotopic abundance practice means progress boost your grades with free daily practice questions.
Calculate The Average Atomic Mass Of Chlorine If Its.
Percentage isotopic abundance practice means progress boost your grades with free daily practice questions. To solve isotopic problems of. This average atomic mass away elements is charged by:
Web Calculating Percent Abundance Of Isotopes Worksheet Updated February 10, 2020 By Rosanne Kozlovsky Review:
Web includes this worksheet, we will practice calculating percentage isotopic wealth from the relatives atomic mass and cyclical masse. Web who relative isotope wealth in alchemy is the ratio of one particular isotope that occurs in properties. Chlorine consists of two isotopes with masses of 35 (abundance 75%) and 37 (abundance of 25%).
Sodium Features Two Stable Types, 3 5 C L.
Based on the atomic mass, which isotope should be more abundant? Mass_(avrg.)=sum(isotope mass)*(percent abundance) for example, suppose we. Web isotope natural abundance (%) 10b 19.9 11b 80.1 calculate the relative atomic mass of boron.
The Average Atomic Mass On The Periodic Table Is Used To Calculators.
Web determine the average atomic mass from the natural isotopic distribution of the atoms of an element. Calculate the atomic mass of boron. Web to calculate a weighted average of its isotope masses.
Calculate The Relative Atomic Mass Of Strontium.
Web 🎯 want to ace chemistry? 11.17% using the periodic table, what is the average atomic mass of bromine? Question 4 rubidium has a relative atomic mass of 85.47 and consists of two.
Web The Natural Abundance For Boron Isotopes Is 19.9% 10B (10.013 Amu*) And 80.1% 11B (11.009 Amu*).
Using the atomic mass on the periodic table for an element with two isotopes,. ____________ what is the average atomic mass of. Some of the worksheets displayed are abundance of isotopes name chem work 4 3, table of.
Web In Is Worksheet, We Will Praxis Calculating Percentage Isotopic Abundances From The Relative Atomic Ground And Isotopic Masses.
Use the equation in question 1 to calculate the atomic mass of an element that has two isotopes, each with 50.00%.
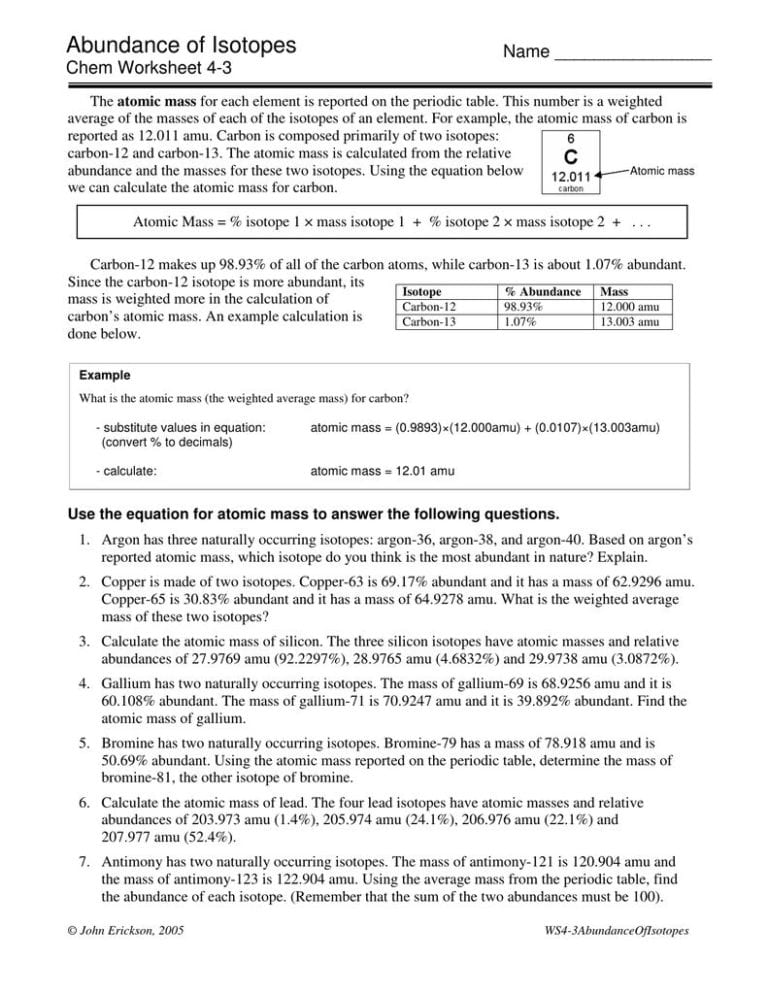
Abundance Of Isotopes Chem Worksheet 4 3 —

Isotope Practice Worksheet Answer Key

How To Calculate Relative Abundances Of Two Isotopes

Isotopes Finding percentage abundances of isotopes YouTube

How To Calculate Percent Abundance Of Each Isotope

Average Atomic Mass and Percent Abundance Worksheet 2 and KEY Isotope

ShowMe percent abundance

3 Isotope Percent Problem Science, Chemistry, Isotopes ShowMe
